
Most of the p-block elements are non-metallic elements with few metalloids and heavy metals. The p-block elements are placed from group-13 to group-18 in the long form of the periodic table. They form ionic compounds with nonmetals and have metallic bonds between metal ions. The valence shell electrons in these elements are placed in s-orbital. The s-block elements are placed in the group-1 and 2. On the basis of presence of valence electrons in an element, elements can be classified as s, p, d and f-block elements.
#PROPERTIES OF A PERIODIC TABLE SERIES#
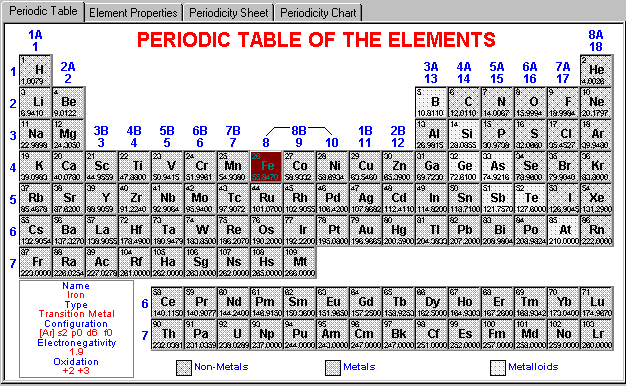
It consists of 18th vertical columns and 7 horizontal periods with two series of 14 elements at the bottom of the main table. He followed the known atomic weight of elements.Īfter the discovery of radioactivity and Noble gases, the Long Form of Periodic Table came in picture. He put all elements, known that time in a certain logical order and also left room for unknown elements. The chemical properties depend on the valence shell electronic configuration whereas physical properties depend on the atomic size mainly.ĭmitrii Mendeleev proposed the first periodic table in 1880 with all known elements. All elements can be differentiated on the basis of their chemical and physical properties.

Differentiating-Trigonometric-Functions.


 0 kommentar(er)
0 kommentar(er)
